Abstract
Introduction: Translocation between chromosomes 4 and 14 [t(4;14)] occurs in 15% of patients with multiple myeloma (MM), and is considered a high-risk (HR) cytogenetic abnormality, associated with inferior outcomes. Induction therapy followed by autologous hematopoietic stem cell transplantation (autoHCT) and prolonged maintenance therapy is considered the standard of care for high risk MM (HRMM) patients, yet there is scarce data on post-transplant outcomes for HRMM patients with t(4:14). Here we report the outcomes of patients with t(4;14) who received an autoHCT at our institution.
Methods: We conducted a retrospective, single-center, chart review analysis of adult MM patients with t(4:14) that was detected by fluorescence in situ hybridization (FISH), who received an autoHCT between 2008-2018. Other HR cytogenetic abnormalities included del (17p), t(14;16) and 1q21 gain or amplification. We evaluated progression free survival (PFS), overall survival (OS), day 100 and best post-transplant responses, and minimal residual disease (MRD) status at best response. MRD status on the bone marrow biopsy was evaluated using 8-color next generation flow cytometry with sensitivity of 1/10-5 cells.
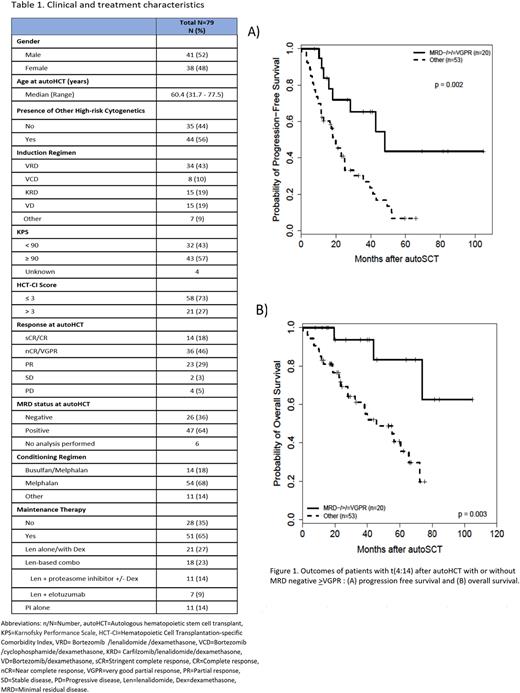
Results: Seventy-nine MM patients with t(4:14) were included in the analysis, with a median age of 60 (range: 32-78) years, and 52% were male. Forty-four (56%) patients had an additional HR cytogenetic abnormality. Overall, 63% (n=50) had achieved ≥VGPR prior to autoHCT, and only 36% (n=26) had MRD negativity at autoHCT. Patient characteristics are presented in Table 1. Median follow up for survivors was 35.7 (range 7.7-111.6) months. Post-transplant, 85% and 91% of patients achieved ≥VGPR at 100-day post-transplant and at final post-transplant evaluation, respectively, whereas 63% achieved MRD-negative status. For the entire cohort, median PFS was 22.9 months, and median OS was 60.4 months. These are inferior outcomes when compared to contemporary outcomes for MM patients after autoHCT, as recently reported by our group (median PFS of 50 months and median OS of 101.7 months; Gaballa et al. Leuk Lymphoma 2022). The presence of an additional HR cytogenetic abnormality with t(4:14) was not associated with inferior PFS (hazard ratio (HR) [95% CI]: 0.86 [0.50-1.47], p=0.57) or OS (HR: 0.87 [0.44-1.73], p=0.70) in the present study.
On univariate analysis (UVA), achieving ≥VGPR before autoHCT was predictive of better PFS (HR: 0.52 [0.30-0.90], p=0.019) and OS (HR: 0.45 [0.23-0.89], p=0.022), which was not observed on multivariable analysis (MVA). In contrast, achieving MRD negative ≥VGPR was associated with a better PFS and OS on both UVA and MVA [(HR: 2.86 [1.32-6.19], p=0.008) and (HR: 9.40 [2.44-36.21], p=0.001), respectively] (Figures 1A and 1B). Lenalidomide (Len)-based combination for post-transplant maintenance was associated with improved OS in both UVA and MVA (HR: 0.08 [0.02-0.34], p<0.001), without a significant impact on PFS (HR: 0.77 [0.38-1.55], p=0.46).
Type of induction was not associated with PFS (p=0.65) or OS (p=0.42), and neither was the conditioning regimen (busulfan+melphalan vs. melphalan) (p=0.31 and p=0.66, respectively).
Conclusions: Patients with MM with t(4:14) have overall poor outcomes, despite novel induction, autoHCT and post-transplant maintenance. Deeper pre-transplant response and post-transplant Len-based combination maintenance were associated with improved outcomes on MVA. Novel immune- and cellular therapies may be used to improve the outcomes in this group of high-risk MM.
Disclosures
Srour:Orca Bio: Research Funding. Nieto:Affimed: Other: Scientific advisory Board, Research Funding; Secura Bio: Research Funding; Astra Zeneca: Research Funding. Lee:Takeda Pharmaceuticals: Consultancy, Research Funding; GSK: Consultancy, Research Funding; Sanofi: Consultancy; Genentech: Consultancy; Monte Rosa Therapeutics: Consultancy; Legend Biotech: Consultancy; Oncopetides: Consultancy; Amgen: Research Funding; Janssen: Research Funding; Pfizer: Consultancy; Regeneron: Research Funding; Immunitas Therapeutics: Consultancy; Karyopharm: Consultancy. Patel:Janssen, Celgene/BMS, Caribou Sciences, Arcellx, Cellectis, Merck, Pfizer, Karyopharm, Oncopeptides: Consultancy. Kebriaei:Pfizer: Consultancy; Amgen: Research Funding; Ziopharm: Research Funding; Kite: Consultancy; Jazz: Consultancy. Thomas:Bristol Myers Squibb: Research Funding; Genentech: Research Funding; Cellectar Pharma: Research Funding; Janssen Pharma: Research Funding; X4 Pharma: Research Funding; Ascentage Pharma: Research Funding. Orlowski:Abbvie, BioTheryX, Inc., Bristol-Myers Squibb, Janssen Biotech, Karyopharm Therapeutics, Inc., Meridian Therapeutics, Monte Rosa Therapeutics, Neoleukin Corporation, Oncopeptides AB, Regeneron Pharmaceuticals, Inc., Sanofi-Aventis, and Takeda Pharmaceutic: Honoraria, Membership on an entity's Board of Directors or advisory committees; CARsgen Therapeutics, Celgene/Bristol Myers Squibb, Exelixis, Janssen Biotech, Sanofi-Aventis, Takeda Pharmaceuticals North America, Inc.: Research Funding; Asylia Therapeutics, Inc.: Current equity holder in private company; Asylia Therapeutics, Inc., BioTheryX, Inc., Heidelberg Pharma, Inc.: Research Funding. Shpall:Navan: Consultancy; Takeda: Patents & Royalties; axio: Consultancy; Affimed: Other: License agreement; Bayer: Honoraria; NY blood center: Consultancy; adaptimmune: Consultancy; Fibroblasts and FibroBiologics: Consultancy. Champlin:Cell Source Inc.: Research Funding; Bluebird: Other: Data Safety Monitoring Board; Omeros: Consultancy; General Oncology: Other: Data Safety Monitoring Board; Johnson &Johnson: Consultancy; Actinium: Consultancy; Kadmon: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal